Back to search resultsSummaryRMgm-808
|
||||||||||
 *RMgm-808
*RMgm-808| Successful modification | The parasite was generated by the genetic modification |
| The mutant contains the following genetic modification(s) | Gene disruption, Introduction of a transgene |
| Reference (PubMed-PMID number) |
Reference 1 (PMID number) : 25941254 |
| MR4 number | |
| top of page | |
| Parent parasite used to introduce the genetic modification | |
| Rodent Malaria Parasite | P. berghei |
| Parent strain/line | P. berghei ANKA |
| Name parent line/clone | P. berghei ANKA 1037m1f1mocl1 (RMgm-32) |
| Other information parent line | P. berghei ANKA 1037m1f1mocl1 (1037cl1; RMgm-32) is a reference ANKA mutant line which expresses GFP-luciferase under control of a schizont-specific promoter. This reference line does not contain a drug-selectable marker (PubMed: PMID: 20019192). |
| top of page | |
| The mutant parasite was generated by | |
| Name PI/Researcher | J. Lin; C.J. Janse; S.M. Khan |
| Name Group/Department | Leiden Malaria Research Group |
| Name Institute | Leiden University Medical Center |
| City | Leiden |
| Country | The Netherlands |
| top of page | |
| Name of the mutant parasite | |
| RMgm number | RMgm-808 |
| Principal name | 1688cl1 |
| Alternative name | ∆pm4 |
| Standardized name | |
| Is the mutant parasite cloned after genetic modification | Yes |
| top of page | |
| Phenotype | |
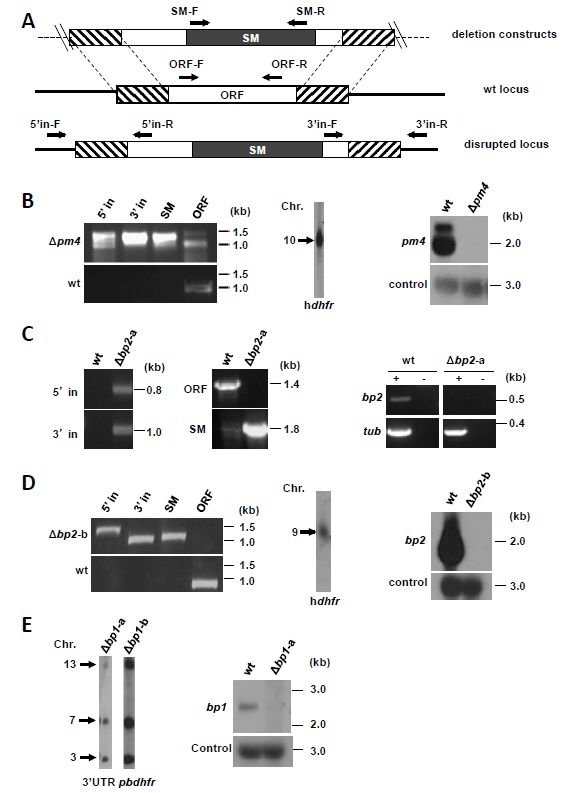
| Asexual blood stage | Asexual blood stages show a (slightly) reduced growth rate and reduced hemozoin formation. |
| Gametocyte/Gamete | Not tested |
| Fertilization and ookinete | Not tested |
| Oocyst | Not tested |
| Sporozoite | Not tested |
| Liver stage | Not tested |
| Additional remarks phenotype | Mutant/mutation
Figure: Generation of the P. berghei mutants ∆pm4 (RMgm-808), ∆bp1 (RMgm-816) and ∆bp2 (RMgm-809). |
 Disrupted: Mutant parasite with a disrupted gene
Disrupted: Mutant parasite with a disrupted gene| top of page | |||||||||||||||||||||||||
| Details of the target gene | |||||||||||||||||||||||||
| Gene Model of Rodent Parasite | PBANKA_1034400 | ||||||||||||||||||||||||
| Gene Model P. falciparum ortholog | PF3D7_1407800 | ||||||||||||||||||||||||
| Gene product | plasmepsin IV | ||||||||||||||||||||||||
| Gene product: Alternative name | PM4, GEXP16 | ||||||||||||||||||||||||
| top of page | |||||||||||||||||||||||||
| Details of the genetic modification | |||||||||||||||||||||||||
| Inducable system used | No | ||||||||||||||||||||||||
| Additional remarks inducable system | |||||||||||||||||||||||||
| Type of plasmid/construct used | (Linear) PCR construct double cross-over | ||||||||||||||||||||||||
| PlasmoGEM (Sanger) construct/vector used | No | ||||||||||||||||||||||||
| Modified PlasmoGEM construct/vector used | No | ||||||||||||||||||||||||
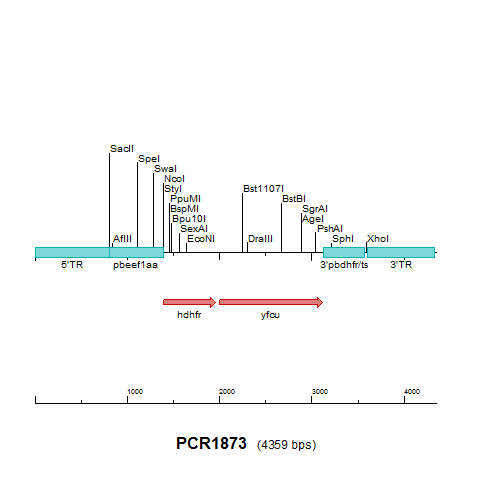
| Plasmid/construct map |
 | ||||||||||||||||||||||||
| Plasmid/construct sequence |
  GAACTCGTACTCCTTGGTGACGTCGCGACCTTGTCGGGGTACTCAGAATTGCAATATATT
| ||||||||||||||||||||||||
| Restriction sites to linearize plasmid | |||||||||||||||||||||||||
| Partial or complete disruption of the gene | Complete | ||||||||||||||||||||||||
| Additional remarks partial/complete disruption | |||||||||||||||||||||||||
| Selectable marker used to select the mutant parasite | hdhfr/yfcu | ||||||||||||||||||||||||
| Promoter of the selectable marker | eef1a | ||||||||||||||||||||||||
| Selection (positive) procedure | pyrimethamine | ||||||||||||||||||||||||
| Selection (negative) procedure | No | ||||||||||||||||||||||||
| Additional remarks genetic modification | The locus was targeted using a linear construct that was generated using a 2-step, anchor tagging PCR method (for primer details see below). The 5’- and 3’ targeting regions of the gene were PCR amplified from genomic DNA using primer pairs 1&2 and 3&4. Primers 2 and 3 have 5’-terminal extensions homologues to the hdhfr::yfcu selectable marker cassette. Primers 1 and 4 both have a 5’-terminal overhang with an anchor-tag which serves as a primer site in the 2nd PCR reaction. The target fragments from the first PCR reaction were annealed to either side of the selectable marker cassette by PCR with anchor-tag primers 5 and 6, resulting in the 2nd PCR product. The hdhfr::yfcu selectable marker cassette used in this reaction was digested from pL0048 using restriction enzymes XhoI and NotI. pL0048 is available from The Leiden Malaria Research Group. See for more information on the 2-step anchor tagging PCR method, the following publications: J.W. Lin, S.M. Khan et al. A novel 'gene insertion/marker out' (GIMO) method for transgene expression and gene complementation in rodent malaria parasites PLoS One. 2011;6(12). T. Annoura et al. Assessing the adequacy of attenuation of genetically modified malaria parasite vaccine candidates Vaccine. 2012;30(16):2662-70 | ||||||||||||||||||||||||
| Additional remarks selection procedure | |||||||||||||||||||||||||
|
Primer information: Primers used for amplification of the target sequences
 Primer information: Primers used for amplification of the target sequences

| |||||||||||||||||||||||||
| top of page | |||||||||||||||||||||||||
 Transgene: Mutant parasite expressing a transgene
Transgene: Mutant parasite expressing a transgene| top of page | |||||||||||||||||||
| Type and details of transgene | |||||||||||||||||||
| Is the transgene Plasmodium derived | Transgene: not Plasmodium | ||||||||||||||||||
| Transgene name | A fusion of GFP (gfp-mu3) and Luciferase Firefly (LucIAV) | ||||||||||||||||||
| top of page | |||||||||||||||||||
| Details of the genetic modification | |||||||||||||||||||
| Inducable system used | No | ||||||||||||||||||
| Additional remarks inducable system | |||||||||||||||||||
| Type of plasmid/construct | (Linear) plasmid double cross-over | ||||||||||||||||||
| PlasmoGEM (Sanger) construct/vector used | No | ||||||||||||||||||
| Modified PlasmoGEM construct/vector used | No | ||||||||||||||||||
| Plasmid/construct map | |||||||||||||||||||
| Plasmid/construct sequence | |||||||||||||||||||
| Restriction sites to linearize plasmid | KspI (SacII) | ||||||||||||||||||
| Selectable marker used to select the mutant parasite | gfp (FACS) | ||||||||||||||||||
| Promoter of the selectable marker | ama-1 | ||||||||||||||||||
| Selection (positive) procedure | FACS (flowsorting) | ||||||||||||||||||
| Selection (negative) procedure | No | ||||||||||||||||||
| Additional remarks genetic modification | The GFP-Luciferase gene (1 copy) has been inserted into the 230p locus (PB000214.00.0) by double cross-over integration. | ||||||||||||||||||
| Additional remarks selection procedure | This reporter mutant expressing GFP-Luciferase does not contain a drug-selectable marker. This mutant has been selected by FACS sorting after transfection based on GFP fluorescence. | ||||||||||||||||||
| top of page | |||||||||||||||||||
| Other details transgene | |||||||||||||||||||
| top of page | |||||||||||||||||||
| Promoter | |||||||||||||||||||
| Gene Model of Parasite | PBANKA_0915000 | ||||||||||||||||||
| Gene Model P. falciparum ortholog | PF3D7_1133400 | ||||||||||||||||||
| Gene product | apical membrane antigen 1 | ||||||||||||||||||
| Gene product: Alternative name | ama-1 | ||||||||||||||||||
| |||||||||||||||||||
| top of page | |||||||||||||||||||
| 3'-UTR | |||||||||||||||||||
| Gene Model of Parasite | PBANKA_0719300 | ||||||||||||||||||
| Gene product | bifunctional dihydrofolate reductase-thymidylate synthase, putative | ||||||||||||||||||
| Gene product: Alternative name | dhfr/ts | ||||||||||||||||||
| |||||||||||||||||||
| Insertion/Replacement locus | |||||||||||||||||||
| Replacement / Insertion | Replacement locus | ||||||||||||||||||
| Gene Model of Parasite | PBANKA_0306000 | ||||||||||||||||||
| Gene product | 6-cysteine protein | ||||||||||||||||||
| Gene product: Alternative name | 230p | ||||||||||||||||||
| |||||||||||||||||||
| top of page | |||||||||||||||||||